DSCSA 2025 Ten Count: Number 8 - FDA and State Inspection Audits, 483, and Warning Letters Increasing
The next highlight you may have missed this year in the DSCSA 2025 Ten Count, list of top DSCSA moments and stories from 2025.


FDA and State Regulatory Agencies Increase DSCSA Specific Inspections, 483s, and Warning Letters
Prompting trading partners to ask if their processes, people, and systems are audit-ready.
With the expiration of most exemptions for DSCSA compliance comes the harsh reality that the necessity to comply with the DSCSA requirements doesn't end when project teams wrap up, and contractors move on to other work. In our regular monthly forums with trading partners, we learned that most large and many smaller organizations have experienced or are expecting FDA and state inspections that include DSCSA requirements. In our webinar in early 2025, we shared that inspections were increasing and public 483s and warning letters were showing that many organizations were finding their DSCSA programs lacking in some important areas. Trading Partners should be working with their quality and regulatory affairs to ensure their SOPs fully address the DSCSA requirements, and their people are trained with documented evidence of training completion. The DSCSA 483 observations and warning letters closely align with the findings from Ten Count's DSCSA mock audits. We continually update our checklists based on new information to assist our clients in effectively demonstrating their compliance. As with any large industry change, the ability to protect patients depends on attention to detail and consistent execution.
In addition to traditional inspections, the FDA has started to leverage Remote Regulatory Assessments (RRAs) to allow for near real time reviews of plants or contract manufacturers in any country. The sites are asked to answer many questions about how they are complying with the DSCSA, as well as providing date-stamped evidence showing the processes in practice. For example, they may ask to see records of handling data exceptions, evidence that associates are trained, and communications used in the event of suspect or illegitimate handling scenarios.
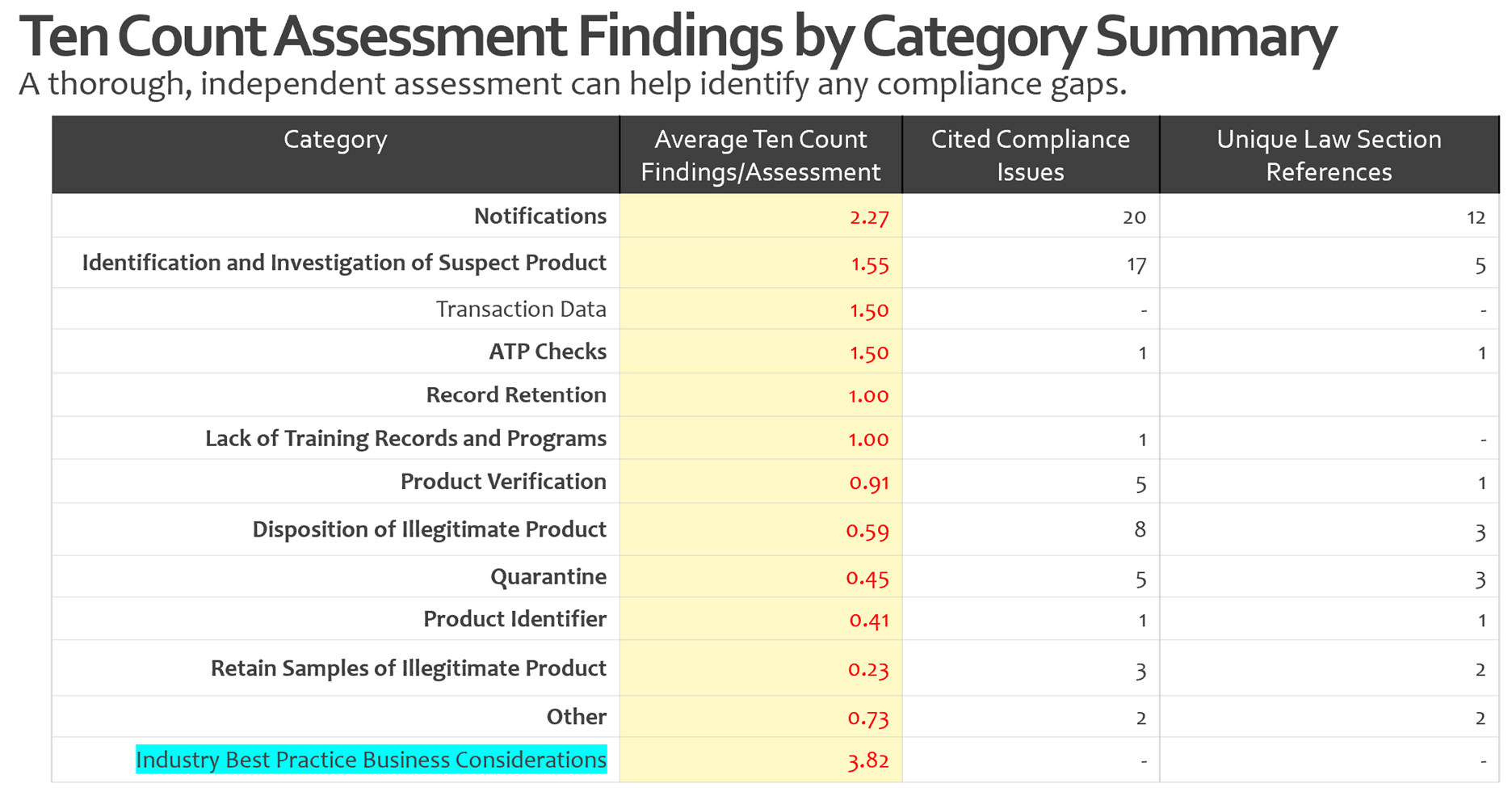
Common DSCSA Compliance Gaps
In our DSCSA compliance assessments, three areas consistently surface as top risk points:
- Handling notifications
- Identification and investigation of suspect or illegitimate products
- Transaction data
We also find that lack of documented training on updated procedures is a common miss that needs to be closely managed. We regularly help clients ensure that their training is comprehensive and updated as things evolve in understanding of compliance and best practices.
We recommend reviewing our free webinar, where we highlighted these typical issues and steps companies can take on their own or with help from a DSCSA experienced consultant.
👉 Access the webinar recording here.
Is your DSCSA compliance where it needs to be? Is your organization looking for industry experts to guide its DSCSA audit readiness efforts? If you’re unsure — or want to strengthen your processes before the FDA shows up — let’s talk. Contact Ten Count Consulting to learn how our DSCSA experts can help protect your business and the patients you serve.




