Another FDA Warning Letter Regarding DSCSA
We reviewed a warning letter that was issued just a few weeks ago to a distributor of pharmaceutical products, or at least that’s the business they were conducting. The findings outlined in this warning letter are quite serious. The company was selling and distributing prescription drugs in addition to its primary business as a medical device distributor. The letter clearly indicates that this company needed to comply with the Drug Supply Chain Security Act (DSCSA), yet they lacked most of the required compliance measures.
The findings in the FDA's warning letter are serious and underscore many common challenges related to DSCSA compliance. Over the past decade, Ten Count Consulting has assisted manufacturers, wholesale distributors, and dispensers in interpreting and implementing the DSCSA. We have conducted numerous DSCSA assessments, and the three most frequent areas of concern align with the findings mentioned in FDA 483's and other warning letters.
Common DSCSA Compliance Gaps
In our DSCSA compliance assessments, three areas consistently surface as top risk points:
- Handling notifications
- Identification and investigation of suspect or illegitimate products
- Transaction data (As the phased exemptions for EDDS expire, we expect to see more FDA findings in this area)
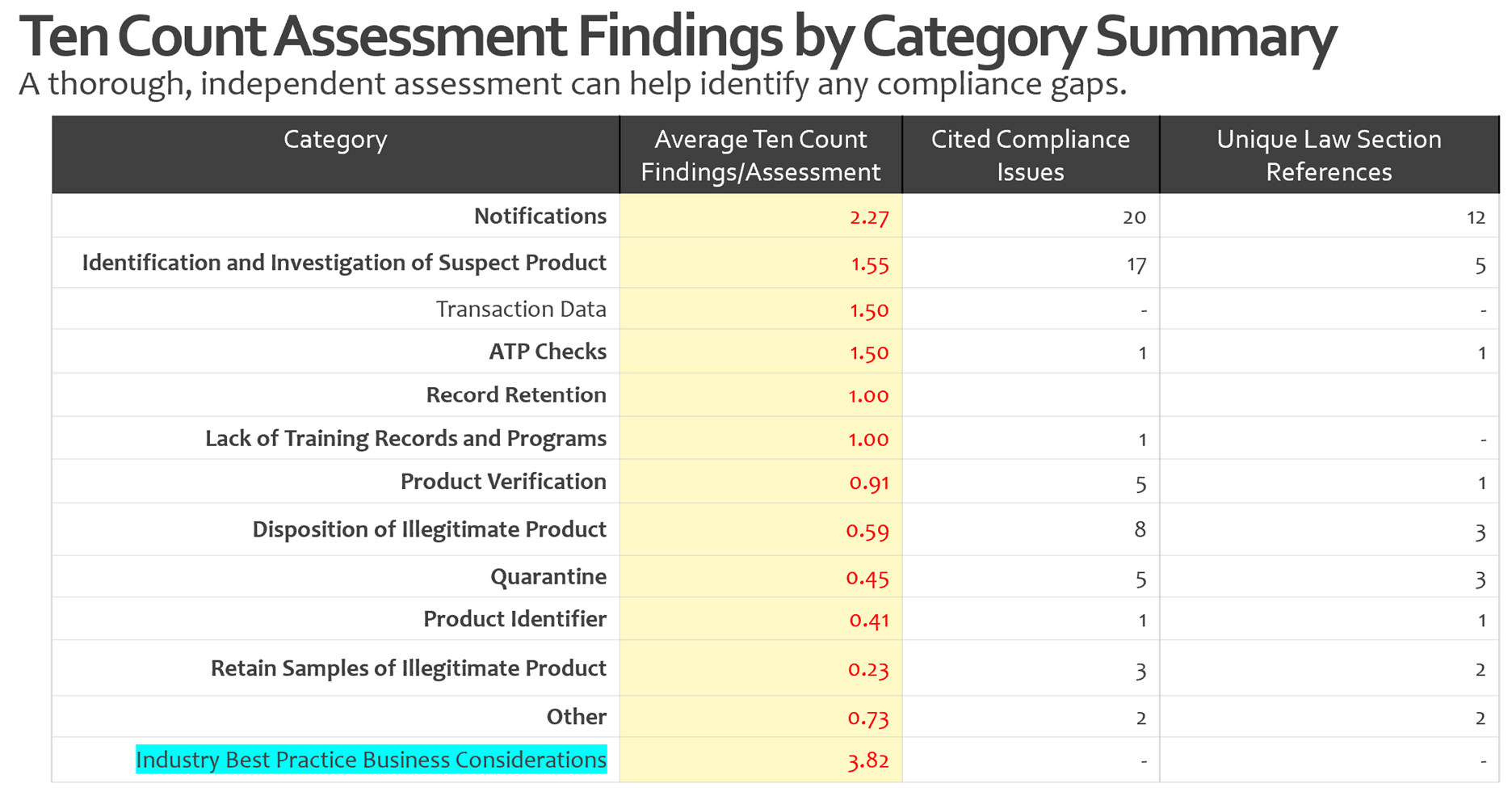
The recent FDA warning letter confirms these pain points, plus several more. We know that operationalizing DSCSA has been a huge challenge for the industry. We understand the complexities that exist in handling the requirements across multiple departments, business units, or work groups.
Compliance: More Than a Serial Number and Data.
Many senior leaders still view the DSCSA as simply a matter of adding serial numbers to bottles and sharing serialized data. However, true compliance with the DSCSA is much more complex. It requires that systems, teams, and procedures work together seamlessly. This complexity can be difficult to understand and even harder to manage.
To provide a clearer overview of these requirements, we have developed a framework that presents a quick, high-level summary. Furthermore, it is crucial to develop, conduct, and document training for all systems, processes, policies, and procedures to ensure ongoing compliance, as illustrated below:
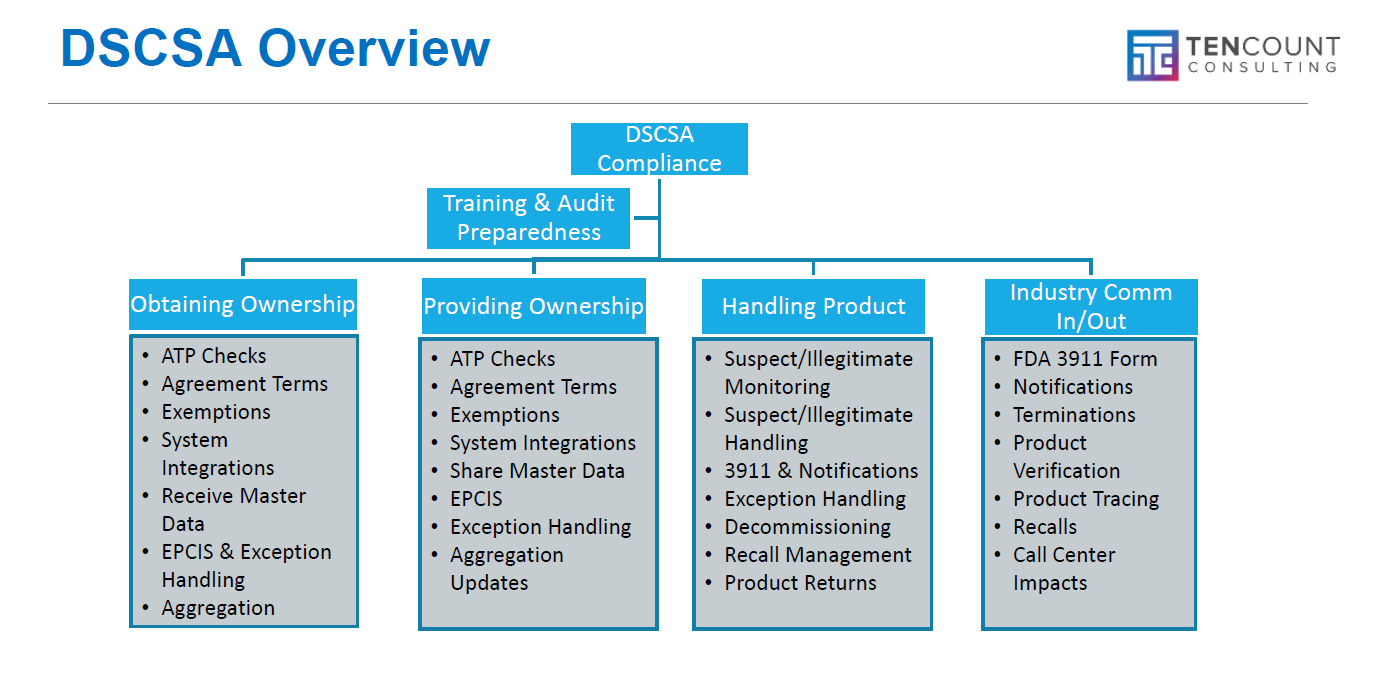
At Ten Count Consulting, we have spent more than a decade:
- Interpreting DSCSA requirements
- Developing practical compliance framework
- Helping companies implement, test, and improve their programs
- Providing tools to maintain compliance and prepare for FDA inspections
Our DSCSA assessments break requirements into manageable parts. We map your processes, identify compliance gaps, and provide actionable steps to close those gaps. Most importantly, we share best practices we’ve seen work well across the industry.
Confidence When the FDA Knocks
Our clients have shared that they are able to use the feedback and tools we provide as a guide during an FDA inspection. They know they can demonstrate the evidence of compliance — and answer inspectors’ questions with clarity and documentation.
If you missed it, we recently hosted a webinar: “Are You Ready to Present Evidence of Your DSCSA Compliance?” In this session, we shared insights from recent 483s, explained how to avoid common oversights, and offered practical advice on preparing for inspections.
👉 Access the webinar recording here.
Is your DSCSA compliance where it needs to be? Is your organization looking for industry experts to guide its DSCSA audit readiness efforts? If you’re unsure — or want to strengthen your processes before the FDA shows up — let’s talk. Contact Ten Count Consulting to learn how our DSCSA experts can help protect your business and the patients you serve.




