Ten Count: HDA Traceability 2025
Top Ten Things We Learned from the HDA Traceability Conference


We were excited to participate in the 2025 HDA Traceability Forum at the Westin in Washington DC on August 4-6. It was a busy conference with lots of networking and note taking and it took us a few weeks to get our summary together. We give you this year's HDA Traceability Ten Count:
- Keep the Main Thing, The Main Thing
It is easy to get lost in all of the DSCSA complexity and loose the spirit that DSCSA is about delivering safe, secure, and high-quality medicine to patients. There are clear requirements to meet but there are also solutions that go beyond required. It does bring potential for innovations but in implementing any new processes or systems, you should be asking how this tangibly advances patient safety, is it widely adopted, and what clear value does it bring.
- NDC-12 Is Approaching
A growing number of industry leaders are raising concern over the level of effort and collaboration needed to address the NDC 12 rule expected to be finalized by FDA by early 2026. This means reviewing all systems, papers, and processes that leverage the existing NDC 10 or GTIN linear UPC barcode to assess potential impact. We believe this effort will require executive level commitment as the risks carry from packaging, to distribution, and all the way through payment and revenue recognition. For most trading partners, this will be much wider than DSCSA.
- Barcode and Data Quality
While the quality of barcodes and data related to DSCSA continues to improve, there are still significant examples of areas that require further improvement. Scannability, durability, and encoded data accuracy of barcodes is an area that manufacturers and packagers should be ensuring are stable and well tested at point of packaging. Serialization data required to be sent to support DSCSA transactional data requirements is typically handled through the use of GS1 EPCIS sent with each shipment or downloadable through portals that may be enabled to share data. Trading partners can leverage conformance checking services from solution providers or may elect to have technical quality teams perform checks. You should always be working with your direct customers to ensure these files are loadable and accurate while expecting to have processes ready to handle exceptions.
- Verification Router Service (VRS) is An Important Tool
Adoption of VRS continues to grow with a significant increase in use by state regulators and dispensers in the past year. VRS is not required specifically but an electronic and interoperable request and response within 24 hours is. Manufacturers should ensure their GTINs are listed in a Lookup Directory or have clearly documented and communicated processes for alternative handling such as email.

- Governance of DSCSA Requires Intentional Focus
DSCSA does not end by implementing all the processes or systems because it must be maintained for the long haul with still evolving guidance, standards, and practices. Most are finding that shared governance across organizations is required for the long haul.
- Master Data & Operational Oversight
Serialization isn’t just IT — it’s a cross-functional responsibility across Quality, Commercial, Supply chain, Trading Partners, — if you can’t align and communicate across partners, your product won’t move.
- Counterfeits and Unsafe Compounding- GLP1s
There is a growing challenge of dangerous products making it to patients due to the spike in demand and short supply of GLP1s that opened up compounding as an alternative for filling patient needs. Now that supply has increased, the FDA has removed from the shortage list but compounders are utilizing an ability to make customized products as an alternative distribution strategy. Note: compounding is a long standing process that can be done safely and does have controls but they are generally less stringent and more flexible by design than full FDA drug approval.
- DSCSA Tools are Helping In Real Suspect/Illegitimate Cases
State Boards of Pharmacy representatives from states shared a real-world example from a multi-state illegal distribution scheme of medications.

- Readiness is Not Perfection, But Proof Is Non-Negotiable
FDA inspections are becoming more thorough and data-driven. Every team from quality to commercial to supply chain must know their procedures, systems and be fully trained. Abha Kundi (ArentFox Schiff and formerly FDA) shared a poem to help remember the important points of readiness for DSCSA. We felt inspired to reform it into the format of a Haiku:
DSCSA Haiku
Process - you do it?
People- you know and use it?
Papers- can prove it?

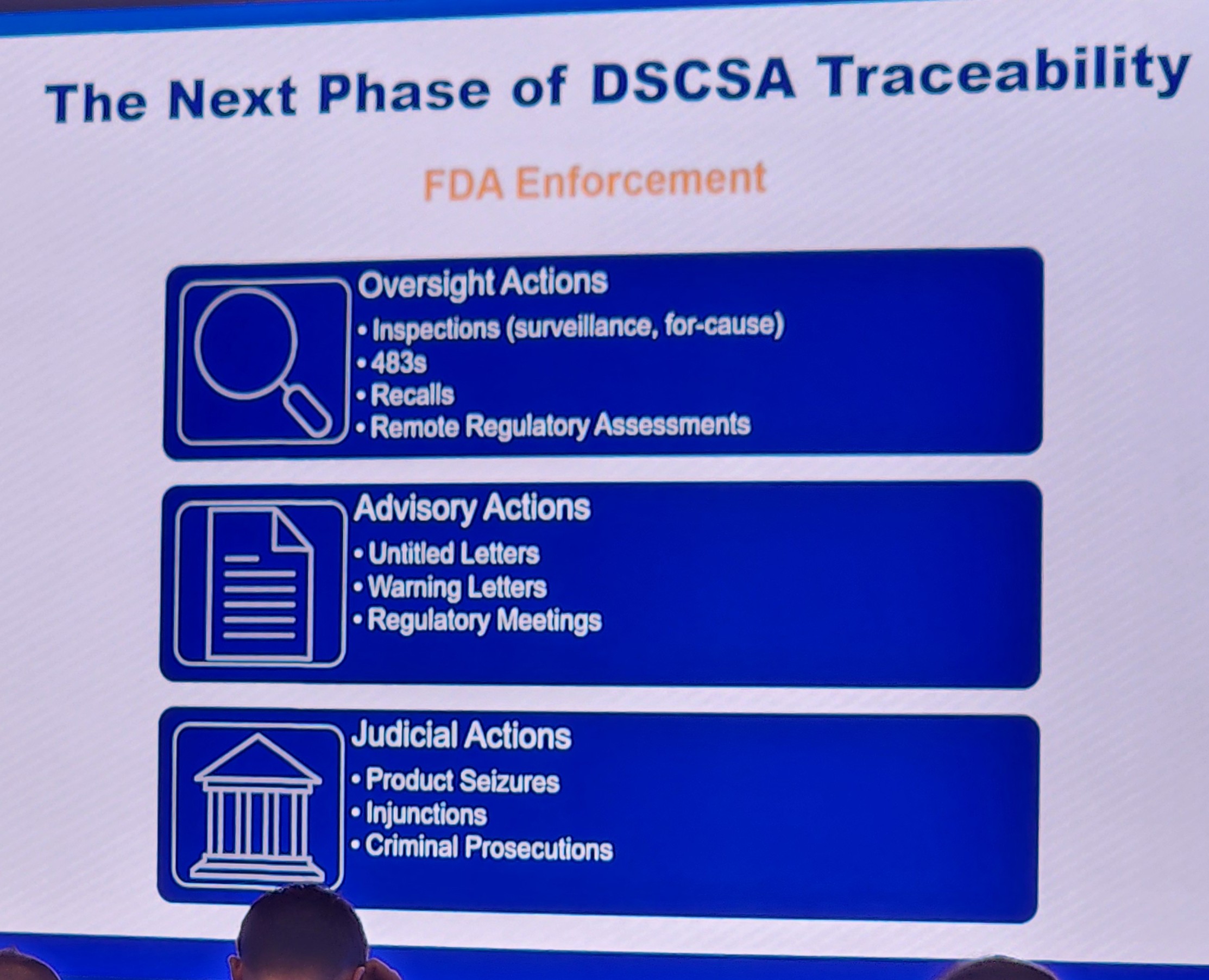
- Compliance Enforcement has arrived
It is clear from all the panels that a new phase of DSCSA has arrived for those sectors who must now fully comply with DSCSA in the manufacturer, repackager, and distributor sectors. Trading partners need to understand that they risk non-compliance, public warning letters, fines, and more if they are not fully prepared for state or federal inspection questions on DSCSA.
Need an Independent Assessment of DSCSA Readiness?
Ten Count's DSCSA readiness assessment and shared governance accelerator helps you rest assured you are fully complying with the law and able to provide evidence in the event of audits. We maintain a comprehensive playbook based on the latest industry insights that can be used to measure readiness and construct a workplan for any areas requiring attention. We leave this playbook as a deliverable so that it can serve your team in keeping compliance evidence up to date and ready for a state or federal audit. We offer a variety of levels of assessments depending on your needs and complexity of business roles.
We would be glad to schedule a free initial consultation to understand your challenges and discuss what type of assessment might make most sense to propose.
Reach us at info@tencountconsulting.com





